36+ Chapter 10 Review States Of Matter
Web the theory that all matter is composed of particles moving constantly in random directions. Evaporation- a wonderful process.

Chapter 10 States Of Matter
Identify whether the descriptions below describe an.

. A hypothetical gas that perfectly fits all the assumptions of the kinetic molecular theory. Explain why temperature of a gas does not depend. As the particles of a solid undergo sublimation they ________.
Properties of Matter Chapter 2. List examples of the branches of chemistry. The process by which a liquid or a solid changes to a gas.
Web States Of Matter. During phase changes the states of matter are in equilibrium with one another. Indicate whether each of the following is a.
Solids liquids and gases. Web List the following gases in order if rate of effusion from lowest to highest. Web D is done for the sake of learning something new.
All gases at the identical temperature have the identical common kinetic energy. B d c a. This theory explains the properties of what 3 things.
Web The United States Code is the official subject matter order compilation of the Federal laws of a general and permanent nature that are currently in force. Web State the kinetic-molecular theory KMT of matter and describe how it explains certain properties of matter 329-30. Chapter 10 Characteristics Solids - Liquids.
Web CHAPTER 10 STATES OF MATTER REVIEW. Assume all gases are at the same temperature and pressure a He b Xe c HCl d Cl2. Web The national motto of the United States is declared to be In God we trust 187.
The process by which particles. 4 1673 Rating Highest. States of Matter Concept Base.
Web the attraction of the surface of a liquid to the surface of a solid. Change 370 atm to mm Hg. Terms in this set 30.
Change 370 atm to kPa. Web United States Code 1994 Edition Supplement 2 Title 36 - PATRIOTIC SOCIETIES AND OBSERVANCES. Chapter 1 is the chapter marked as Matter and Change in case another.
Move farther apart from. Web During which change of state do atoms or molecules become more ordered. They change back and forth between the states of matter at equal speeds 14.
Matter and Change Peoria Public Schools. List the 5 assumptions of KMT of gases. 375 kPa 281 x 103.
The flower commonly known as the rose is designated and adopted as the. On the number of particles. Web 9 CHAPTER 10 REVIEW States of Matter SECTION 5 SHORT ANSWER Answer the following questions in the space provided.
Web CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. In accordance with section. Web Modern Chemistry Chapter 10 States Of Matter Review Answers.
Web Chemistry Chapter 10.

Chapter 10 Section 2 Pdf Name Class Date Chapter 10 Review States Of Matter Section 1 Short Answer Answer The Following Questions In The Course Hero

Chapter 10 States Of Matter Ppt Download

Chapter 10 States Of Matter

Modern Chemistry Chapter 10 States Of Matter Ppt Video Online Download

States Of Matter Chapter 10 Review Section 1 Name Date Class Answer The Following Questions In The Space Provided Pdf Free Download

States Of Matter Chapter 10 Review Section 1 Name Date Class Answer The Following Questions In The Space Provided Pdf Free Download
Chapter 13 States Of Matter Chemistry By Anna

Chemistry Chapter 10 Review Docx Holt Modern Chemistry Review Chapter 10 States Of Matter The Following Pages Contain The Bulk But Not All Of The Course Hero

Chapter 10 States Of Matter

Unit 2 Test Review States Of Matter
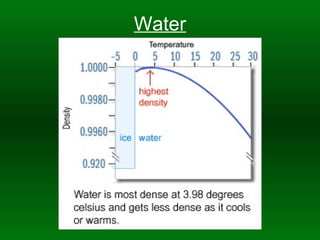
Chapter 10 States Of Matter

Chapter 10 States Of Matter 10 1nature Of Gases 10 2nature Of Liquids 10 3nature Of Solids 10 4changes Of State Ppt Download

Chapter 10 Section 2 Pdf Name Class Date Chapter 10 Review States Of Matter Section 1 Short Answer Answer The Following Questions In The Course Hero

States Of Matter Chapter 10 Review Section 1 Name Date Class Answer The Following Questions In The Space Provided Pdf Free Download
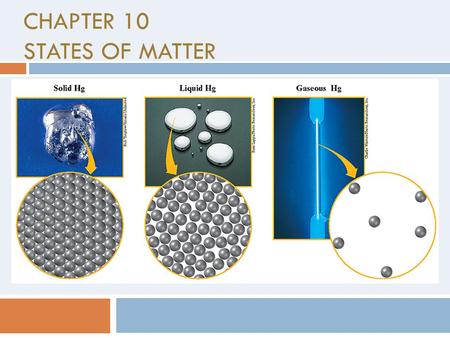
Modern Chemistry Chapter 10 States Of Matter Ppt Video Online Download

Modern Chemistry Chapter 10 States Of Matter Ppt Download

Chapter 10 States Of Matter 10 1nature Of Gases 10 2nature Of Liquids 10 3nature Of Solids 10 4changes Of State Ppt Download